
NBS superfood: یک مکمل امیدوارکننده
1403/01/10 مقالات علمی
NBS superfood: a promising adjunctivetherapy in critically ill ICU patients with omicron variant of COVID-19
Mehrdad Mosadegh1, Aref Khalkhali2, Yousef Erfani3, Manije Nezamdoost4, Seyyed Hamid Hashemi5, Farid Azizi Jalilian6,7*, Nastaran Ansari6, Shahab Mahmoudvand6, Mojgan Mamani5, Elham Abdoli5, Razieh Amini7 and Gholamreza Kalvandi8
Abstract
This clinical trial aimed to assess the impact of Nutrition Bio-shield superfood (NBS) on clinical status among critically ill ICU patients suffering from acute respiratory distress syndrome (ARDS) due to the Omicron variant of COVID-19.
A total of 400 patients with confirmed Omicron-related ARDS were randomly assigned to either the intervention group (n = 200) or the control group (n = 200). Patients in the intervention group received 1.5 g of NBS powder daily for 2 weeks in addition to standard antiviral treatment, while the control group received a placebo alongside standard antiviral therapy. Serum samples were collected from all patients in both groups, and various clinical and laboratory parameters, including ESR, CRP, D-Dimer, CPK, WBC count, lymphocyte count, and lymphocyte percentage, were measured using established methodologies. Following a 14-day intervention period, the intervention group exhibited a significant reduction in mean serum levels of CRP (15.39 vs. 48.49; P < 0.001), ESR (14.28 vs. 34.03; P < 0.001), D-Dimer (485.18 vs. 1009.13; P = 0.001), and CPK (68.93 vs. 131.48; P < 0.001) compared to the control group. Conversely, a sig- nificant increase was observed in the mean serum levels of lymphocytes (1537.06 vs. 1152.60; P < 0.001) in the inter- vention group after 14 days of treatment compared to the control group. The remarkable reduction in inflammatory markers and mortality rates observed with NBS supplementation alongside standard antiviral treatment underscores its crucial role in mitigating inflammation and achieving an important milestone in the fight against COVID-19.
Keywords Nutrition bio-shield, SARS-CoV-2 Omicron variant, COVID-19, Complementary therapies, Respiratory distress syndrome
*Correspondence:
Farid Azizi Jalilian
1 Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
2 Department of Science, Faculty of Biology, Islamic Azad University, Mashhad, Iran
3 Department of Medical Laboratory Sciences, School of Allied Medical Sciences, Tehran University of Medical Sciences, Tehran, Iran
4 Department of Internal Medicine, Division of Infectious Disease, Farabi Hospital, Social Security Organization, Mashhad, Iran
5 Department of Infectious Diseases, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
6 Department of Virology, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
7 Research Center for Molecular Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
8 Department of Pediatrics, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2), initially documented on December 31, 2019, has been identified as the etiological agent responsible for the onset of the infectious ailment recognized as coronavi- rus disease 2019 (COVID-19) (Azizi Jalilian et al. 2022). COVID-19, having disseminated globally, attained the status of a global pandemic as officially declared by the World Health Organization (WHO) on March 11, 2020 (Weidmann et al. 2021; Oristrell et al. 2022). Accord- ing to the data released by the WHO, as of February 4, 2024, the cumulative count of confirmed COVID-19 cases stands at 774,593,066, accompanied by a total of 7,028,881 recorded fatalities. Furthermore, as of August 5, 2023, a cumulative total of 13,492,225,267 vaccine doses has been globally administered (https://covid19. who.int/). In Iran, as of August 5, 2023, a cumulative total of 155,441,243 vaccine doses has been administered. Nonetheless, despite widespread vaccination efforts, from January 3, 2020, to February 18, 2024, the COVID- 19 pandemic has persisted with an unsettling toll, reg- istering over 7,626,230 confirmed cases and surpassing 146,785 recorded fatalities (https://covid19.who.int/ region/emro/country/ir).
Research findings have elucidated that individuals aged over 65 years, those afflicted by chronic obstructive pul- monary disease, diabetes mellitus, chronic renal disease, malignant tumors, hyperlipidemia, hypertension, and post-transplantation immunodeficiency, constitute a cohort vulnerable to the severe manifestation of the dis- ease (Gadotti et al. 2020; Azimi et al. 2021).
Patients afflicted by SARS-CoV-2 typically present with dual clinical profiles. In the majority, constituting 75 to 80% of cases, the disease manifests as a spectrum of symptoms spanning mild to moderate, encompassing manifestations such as fever, chills, fatigue, cough, sore throat, and alterations in taste and/or olfaction. Con- versely, a subset comprising 10 to 15% of patients mani- fests a severe form of the ailment (Leulseged et al. 2021; Legacy et al. 2022). In the severe form of the disease, patients commonly exhibit notable abnormalities, includ- ing pulmonary infiltrates, multi-organ dysfunction, and declining oxygen saturation levels. Typically, individuals with these manifestations necessitate hospitalization in tertiary care facilities (Azimi et al. 2021). Furthermore, it has been observed that in the context of severe COVID-
19 pathology, SARS-CoV-2 can instigate a cascade of events, characterized by hyperactivation of the immune system, disproportionate release of proinflammatory cytokines, heightened oxidative stress, and the activation of pro-coagulatory factors (Mortaz et al. 2021).
Laboratory findings have demonstrated that patients experiencing the severe form of COVID-19 infection
exhibit elevated neutrophil counts and increased lev- els of various factors, including serum C-reactive pro- tein (CRP), interleukin-1 beta (IL-1β), IL-2, IL-6, IL-7, IL-8, IL-10, and IFN gamma associated with TH1 response. Conversely, their blood samples indicate a sig- nificant reduction in platelet and lymphocyte counts. It is widely acknowledged that the immune response to SARS-CoV-2, along with the secretion of inflammatory cytokines, plays a pivotal role in the progression of the infectious disease (Speakman et al. 2021; Mosadegh et al. 2022a, b).
Dietary supplements play a valuable role in modulating and supporting the functioning of the immune system. Extensive research has shown that vitamins, miner- als, and herbs have the potential to mitigate the dura- tion and severity of viral infections, including influenza (Beigmohammadi et al. 2021; Gönen et al. 2021). The Nutrition Bio-Shield (NBS Organik®, İstanbul, Turkey) powder is designated with an approved identification number (006633-14.11.2019) and is categorized as a nat- ural herbal supplement known for its immune system- enhancing properties (Bayat et al. 2014). NBS primarily
derives from wheat germ and encompasses a diverse array of essential vitamins, including A, B1–B3, B5, B6, B9, C, D, K, and a complement of vital minerals such as potassium, manganese, magnesium, phosphorus, sul- fur, boron, calcium, iron, zinc, copper, as well as essen- tial fatty acids like omega-6 and omega-9, in addition to other macro and micro molecules (Azizi Jalilian et al. 2022; Mosadegh et al. 2022a, b).
Currently, a variety of vaccines are available for the pre- vention and treatment of COVID-19 infection (Le et al. 2020, Tzenios et al. 2023). However, in response to the emergence of novel variants of the SARS-CoV-2 virus, the imperative task of designing and conducting clini- cal trials to identify effective and innovative therapeutic interventions becomes increasingly vital. The aim of this clinical trial study is to assess the impact of NBS pow- der on the clinical condition and inflammatory markers in critically ill intensive care unit (ICU) patients afflicted with acute respiratory distress syndrome (ARDS) attrib- uted to the Omicron variant of COVID-19.
Materials and methods
Ethics approval and trial registration
The study protocols adhered to the principles out- lined in the Helsinki Declaration, with all procedures receiving official validation from the Ethics Commit- tee of Hamadan University of Medical Sciences (Pro- tocol Number: IR.UMSHA.REC.1401.1043; Approval Date: 2023-03-04). Additionally, the study’s protocol has been registered in the Iranian Registry of Clinical Tri- als (IRCT), accessible at www.irct.ir (IRCT Registration
Number: IRCT20230116057135N2; Registration Date: 2023-04-05).
Study design and participants
The current investigation employed a rigorous double- blind, randomized clinical trial design conducted at Far- abi Hospital in Mashhad, Iran. The objective of this study was to assess the impact of NBS on the clinical condition and mortality rate among critically ill ICU patients suf- fering from ARDS attributed to the Omicron variant of COVID-19. The study’s participants were exclusively drawn from the pool of individuals admitted to the ICU at Farabi Hospital, each of whom had received a defini- tive diagnosis of the Omicron variant of COVID-19. A total of 400 patients were enrolled in the study, with 200 patients assigned to the intervention group and another 200 to the control group (Fig. 1). The informed written consent was meticulously obtained from all enrolled patients, ensuring their voluntary participation in the study.
Inclusion criteria
Inclusion criteria for the current study encompassed patients who fulfilled the following conditions: (1) will- ingness to participate, as demonstrated by the signing of written informed consent, (2) a confirmed diagnosis of the Omicron variant of COVID-19, accompanied by ARDS, (3) admission to the ICU setting, (4) absence of underlying comorbidities, and (5) inclusion of both male and female patients. The definitive confirmation of the Omicron variant of COVID-19 was established through
a two-tiered diagnostic process involving Reverse Tran- scriptase-Polymerase Chain Reaction (RT-PCR) assay, which included the retrieval of RT-PCR Ct values, and subsequent genome sequencing analysis.
Exclusion criteria
Patients were excluded from participation in the present study based on the following criteria: (1) prior involve- ment in other clinical trial studies, (2) non-consent and absence of signed informed consent, (3) testing negative for the Omicron variant of COVID-19, (4) manifestation of ARDS attributed to alternative viral infections, such as human immunodeficiency virus, Hepatitis C, Hepatitis B, or other common respiratory viruses, (5) presence of underlying medical conditions or history of solid organ or hematological transplantation, (6) pregnancy, and (7) consumption of supplementary substances within the three-month period preceding the commencement of the study.
Interventions and randomization
The study enrolled a total of 400 patients who presented with ARDS and received a confirmed diagnosis of the Omicron variant of COVID-19. Employing a randomized allocation method, all patients were subsequently divided into two distinct groups: (1) the intervention group (n = 200) and (2) the control group (n = 200).
In the intervention group, patients received a specific treatment regimen comprising the NBS superfood in addi- tion to standard antiviral therapy. This entailed the admin- istration of 4.5 g of NBS powder daily, distributed across
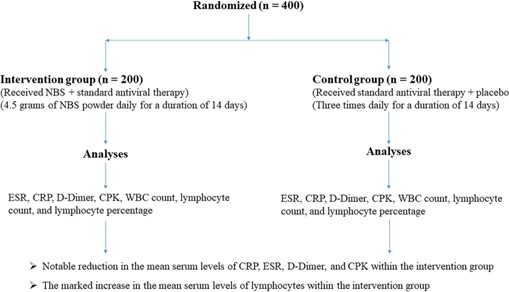
Fig. 1 Consort flow chart
three equal doses: 1.5 g in the morning (6 a.m. to 12 p.m.),
1.5 g in the afternoon (12 p.m. to 6 p.m.), and 1.5 g in the evening (6 p.m. to 12 a.m.), administered over a duration of 2 weeks. Patients allocated to the control group received standard antiviral therapy alongside a placebo. The pla- cebo was administered three times daily for a duration of 14 days. This regimen was devoid of any active ingredi- ents, serving as an inert comparator. The standard antivi-
ral treatment protocol encompassed the administration of Kaletra (Lopinavir + Ritonavir) (AbbVie Inc., North Chi- cago, Illinois, U.S.A) in combination with Hydroxychloro- quine (Oxiklorin, Elyson Pharmaceutical Co. Ltd., Seoul,
Korea). It is pertinent to note that the ICU specialists were informed of the intervention allocation for both study groups, while patients enrolled in both the intervention and control groups as well as nurses remained blinded to their respective treatment assignments.
Clinical and laboratory biomarkers measurements
Blood samples, totaling 10 ml (comprising 5 ml at the out- set of the study and an additional 5 ml following the inter- vention), were meticulously collected from all patients enrolled in both the intervention and control groups. These samples were collected to facilitate the assessment of clinical and laboratory parameters. All blood samples were acquired utilizing standard coagulation tubes and subsequently subjected to centrifugation at 10,000 revolu- tions per minute (rpm) for a duration of 10 min to facilitate serum isolation. Subsequently, the quantification of clinical and laboratory parameters, including Erythrocyte Sedi- mentation Rate (ESR), serum CRP, D-Dimer (a fibrin deg- radation product), Creatine Phosphokinase (CPK), White Blood Cell (WBC) count, lymphocyte count, and lym- phocyte percentage, was executed through the application of established kits and methodologies. These assessments were conducted both at baseline (prior to intervention ini- tiation) and on the 14th day following the intervention.
Statistical analysis
All clinical attributes and laboratory findings were metic- ulously incorporated into SPSS version 23.0 (SPSS Inc., Chicago, IL, USA). Comprehensive data analysis was conducted employing a battery of statistical tests, includ- ing independent samples t-test, paired t-test, and the chi-square test, as appropriate for the specific data charac- teristics and objectives of the study.
Results
Comparison of clinical characteristics and laboratory results in both control and intervention groups
In the intervention group, the mean age of all patients was 51.33 ± 14.84 years, with an age range spanning from 22 to 93 years. Conversely, in the control group, the
median age was 51.74 ± 13.01 years, ranging from 26 to 83 years.
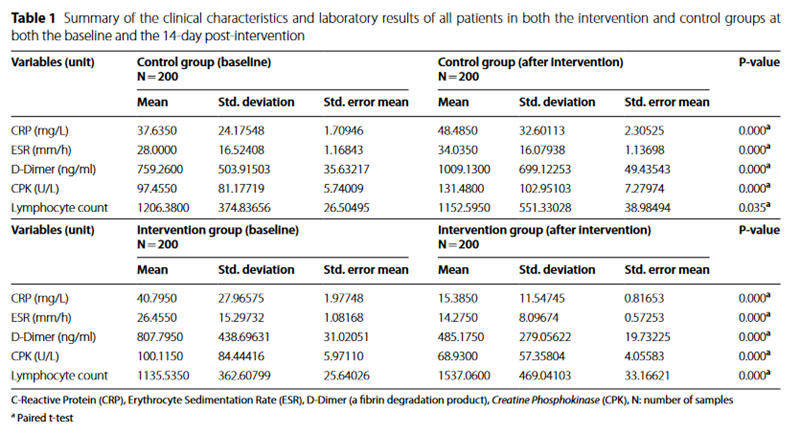
Table 1 provides a comprehensive summary of the clinical characteristics and laboratory results of all patients in both the intervention and control groups at both the baseline and the 14-day post-intervention time points. Significant statistical differences were observed in the levels of CRP (P < 0.001), ESR (P < 0.001), D-Dimer (P < 0.001), and CPK (P < 0.001) in the control group 14 days following the administration of placebo alongside standard antiviral treatment. The findings indicated an increase in CRP, ESR, D-Dimer, and CPK levels at the 14-day post-standard antiviral treatment interval.
In contrast, our analyses unveiled a substantial decrease in the mean levels of CRP (P < 0.001), ESR (P < 0.001), D-Dimer (P < 0.001), and CPK (P < 0.001) within the intervention group, observed 14 days subsequent to the administration of NBS alongside standard antiviral treat- ment. Furthermore, a noteworthy increase in the mean lymphocyte count (P < 0.001) was documented 14 days post-consumption of NBS superfood.
Comparison of variables in both control and intervention groups at the baseline and after a 14‑days interval
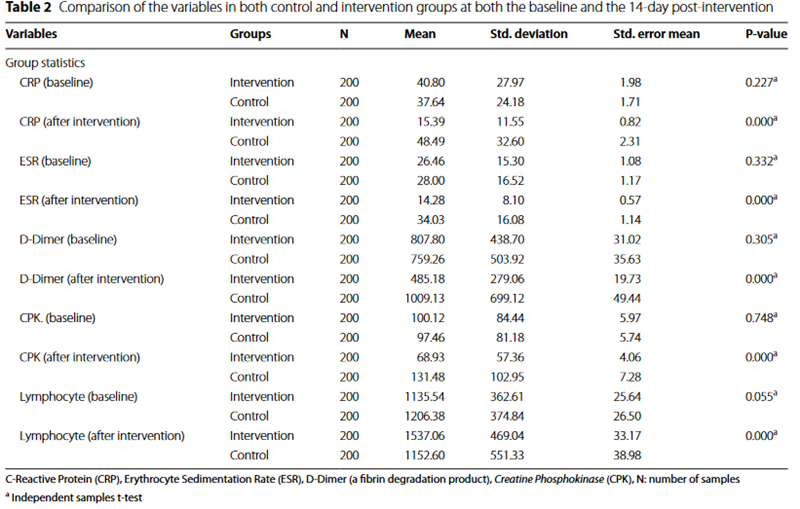
Table 2 provides a concise summary and comparative analysis of the clinical attributes and laboratory results for all patients enrolled in both the intervention and control groups, both at the study’s outset and 14 days following the intervention. The comparative analysis of the investigated variables between the control and inter- vention groups demonstrated a notable reduction in the mean serum levels of CRP (15.39 versus 48.49; P < 0.001), ESR (14.28 versus 34.03; P < 0.001), D-Dimer (485.18 ver-
sus 1009.13; P = 0.001), and CPK (68.93 versus 131.48;
P < 0.001) at the 14-day post-intervention juncture within the intervention group, in stark contrast to the control group. Conversely, a marked increase was observed in the mean serum levels of lymphocytes (1537.06 versus 1152.60; P < 0.001) 14 days following the intervention within the intervention group, compared to the control group.
We did not find significant differences in the mean of serum levels of CRP (P = 0.227), ESR (P = 0.332), D-Dimer (P = 0.305), and CPK (P = 0.748) at baseline between intervention and control groups.
The effects of NBS consumption on surveyed variables
To evaluate the association between the percentage of patients transitioning from abnormal to normal values for various parameters within both the intervention and control groups, we employed the Chi-square (χ2) test. Our analysis yielded statistically significant differences in the proportions of patients achieving this transition
between the two cohorts. For CRP, it is noteworthy that 27.5% of patients in the intervention group and a mere 17.5% in the control group exhibited a transition from abnormal to normal values, with a remarkably low P-value of 0.000. In the case of ESR, a substantial dis- parity is evident, as 43% of patients in the intervention group and only 5% in the control group experienced a transition from abnormal to normal values (P = 0.000). With regard to D-Dimer, 42.5% of patients in the inter- vention group and a mere 8.3% in the control group suc-
cessfully achieved a transition from abnormal to normal values, signifying a statistically significant difference with a P-value of 0.000. Lastly, for CPK, 18% of patients in the intervention group and merely 10.5% in the control group demonstrated a transition from abnormal to nor- mal values, with a significant P-value of 0.000.
The mortality rate in both the control and intervention groups
Overall, the study revealed mortality rates of 31% (n = 62/200) and 8.5% (n = 17/200) within the control and intervention groups, respectively, with a statistically sig- nificant disparity (P = 0.001), underscoring the substantial impact of NBS consumption in reducing patient mor- tality, as delineated in Table 3. Furthermore, upon con- ducting a more detailed analysis within the intervention group, it was ascertained that 10.3% (n = 9/87) and 7.1% (n = 8/113) of deceased patients were male and female, respectively. Importantly, this analysis did not reveal any statistically significant differences (P = 0.412) between the genders in terms of mortality outcomes. Conversely, within the control group, it was observed that 25.9% (n = 21/81) and 34.55% (n = 41/119) of deceased patients were female and male, respectively, with no statistically significant distinction noted (P = 0.201). Cumulatively, the results indicated that among all patients in both the control and intervention groups, 38.7% (n = 67/173) of deceased patients were aged 50 years or older, and this difference was statistically significant (P = 0.001).
Discussion
The challenges of treating COVID-19 in the face of dif- ferent variants involve considerations of reduced vaccine effectiveness, increased transmissibility, potential resist- ance to treatments, and the need for ongoing surveillance and adaptation of countermeasures to combat the evolv- ing virus(Planas et al. 2021; Tegally et al. 2021; Wang et al. 2021). These challenges highlight the importance of a multidisciplinary approach involving research, public health measures, and healthcare interventions to manage the pandemic effectively. The primary focus of this study, as well as many recent research endeavors, is to inves- tigate the efficacy of suggested primary and adjunctive
therapies as alternatives to target-specific treatments, given the reasons outlined above.
Five distinct variants (Alpha, Beta, Gamma, Delta, and Omicron) of SARS-CoV-2 have been recognized as significant global public health concerns (Meo et al. 2021). The most recent variant, Omicron, was initially identified on November 24, 2021, in South Africa and Botswana, and was subsequently designated as a novel variant by the WHO (Fan et al. 2022). The elevated number of mutations present in the Omicron variant of SARS-CoV-2 has the potential to impact the efficacy of existing vaccines and treatment protocols (Kandeel et al. 2022). As highlighted earlier, the Omicron variant poses a potential challenge to global endeavors aimed at con- trolling SARS-CoV-2 infections. Consequently, there is a compelling need for the meticulous design and execution of comprehensive clinical trial studies to identify effective and innovative therapeutic agents.
The objective of the present study was to assess the effi- cacy of NBS powder, a natural supplement, on the clini- cal condition and inflammatory markers of critically ill ICU patients suffering from ARDS attributed to the Omi- cron variant of COVID-19. Prior to the study, the defini- tive diagnosis of the Omicron variant of COVID-19 had been established through molecular-based testing and sequencing assays.
The comparative analysis within the control group exhibited a notable and statistically significant augmenta- tion in the mean serum levels of CRP, ESR, D-Dimer, and CPK at the 14-day post-intervention, compared to the baseline. In the presence of inflammation, hepatic syn- thesis of CRP occurs, leading to its release into the blood- stream. Additionally, the ESR serves as an indicator of systemic inflammation within the circulatory system. The manifestation of pain, swelling, and erythema in affected tissues can be attributed to the production of CRP and elevation in ESR levels, commonly observed in the con- text of chronic diseases or autoimmune disorders (Spros- ton and Ashworth 2018). Inflammation can be triggered by a spectrum of conditions, encompassing bacterial or viral infections, arthritic disorders, vasculitis, and inflam- matory bowel disease (Ahmed 2011). Elevated results in both the CRP and ESR tests are indicative of systemic inflammation within the body (Sproston and Ashworth 2018). Furthermore, D-Dimer has emerged as a notewor- thy prognostic indicator for assessing the severity and mortality risk associated with COVID-19. Thromboem- bolic complications have been associated with elevated D-dimer levels, a phenomenon attributed not only to such complications but also to acute lung injury in the context of COVID-19 infection (Yao et al. 2020). CPK, an enzyme, naturally occurs in the body and is predomi- nantly localized in the heart, brain, and skeletal muscle
tissues. Elevated blood levels of CPK are indicative of injury or stress affecting the heart, brain, or muscle tis- sues (Kuwahara et al. 2010). Alterations in lymphocyte levels, characterized by increases and decreases, are clini- cally referred to as lymphocytosis and lymphocytopenia, respectively (Shanafelt et al. 2009). The findings of this study unveiled a notable decrease in mean serum lym- phocyte levels at the 14-day post-standard antiviral treat- ment assessment within the control group, in comparison to the baseline measurements. Lymphocytes represent a pivotal component of the body’s immune system, tasked with combating foreign bacteria and viruses. Deviations in lymphocyte levels within the bloodstream, whether elevated or reduced, may bear relevance to underlying inflammatory processes (Jafarzadeh et al. 2021).
Conversely, our analyses unveiled a substantial decrease in the mean levels of CRP, ESR, D-Dimer, and CPK within the intervention group at the 14-day interval sub- sequent to NBS powder administration alongside stand- ard antiviral treatment. A comparative assessment of the surveyed variables between the control and intervention groups showcased a statistically significant reduction in mean serum levels of CRP, ESR, D-Dimer, and CPK, at the 14-day post-intervention period within the interven- tion group, in contrast to the control group. This study unequivocally underscores the potent impact of a 14-day regimen of NBS consumption on the meticulous modu- lation of pivotal inflammatory markers within the body. These results not only corroborate the robust efficacy of NBS but also elucidate its pivotal role in significantly attenuating the levels of CRP, ESR, CPK, and D-Dimer. This effect is instrumental in alleviating life-threatening conditions in patients, as evidenced by recent research findings (Mehta et al. 2020; Ruan et al. 2020; Zhou et al. 2020). Emerging evidence suggests that the mechanism of action underlying this profound effect lies in NBS’s ability to mitigate the excessive release of proinflamma- tory cytokines, curtail oxidative stress, and counteract the activation of pro-coagulation factors, all of which contribute to the amelioration of inflammation and, consequently, improved disease severity and reduced mortality rates among COVID-19 patients. These com- pelling findings emphasize the potential of NBS as an invaluable adjunctive therapy in the battle against severe COVID-19.
The impact of NBS powder on disease severity and immune system function in COVID-19 patients has been investigated in several rigorous scientific studies. In a clinical trial conducted by Jalilian et al. in Iran, the impact of NBS on immune system function in COVID-
19 patients was thoroughly investigated. Their study revealed that the consumption of NBS powder over a duration of 4 weeks yielded a significant influence on the
levels of proinflammatory cytokines, particularly inter- leukin-6 (IL-6) and tumor necrosis factor α (TNF-α), in individuals afflicted with COVID-19 (Azizi Jalilian et al. 2022). Furthermore, Mosadegh et al. conducted a metic- ulously designed study investigating the impact of NBS superfood on a spectrum of laboratory biomarkers and disease severity in patients afflicted with COVID-19. The findings derived from their rigorously executed clinical trial unequivocally demonstrated a significant decrease in the mean serum levels of key parameters, including CRP, ESR, D-Dimer, lactate dehydrogenase (LDH), aspar- tate aminotransferase (SGOT), serum glutamic pyruvic aminotransferase (SGPT), alkaline phosphatase (ALP), WBC count, IL-6, and TNF-α, at the 14-day interval fol- lowing intervention compared to baseline within the intervention group (Mosadegh et al. 2022a, b). Another investigation revealed that the NBS supplement, admin- istered orally to rats with rheumatoid arthritis, signifi- cantly reduced serum levels of inflammatory markers such as ESR and CRP. Moreover, it effectively normalized rheumatoid factor (RF) levels, indicating its potential to alleviate RA symptoms. These findings corroborate the anti-inflammatory properties of NBS observed in clini- cal trials on COVID-19 patients, underlining its broader therapeutic potential across inflammatory conditions. Further research is warranted to solidify its clinical value, but these collective results emphasize the promise of NBS as an adjunctive therapy for inflammatory diseases (Mosadegh et al. 2022a, b). These results underscore the significant impact of NBS superfood in mitigating inflam- mation and ameliorating disease severity among COVID- 19 patients, in alignment with the findings of the present scientific research. The evaluation of disease severity and inflammatory biomarkers among COVID-19 patients has been a subject of investigation by various research- ers, who have explored the potential effects of herbal sup- plements and pharmacological interventions. In a study conducted by Emadi et al. in Iran, the oral administration of Imatinib in hospitalized adults with COVID-19 was meticulously examined to assess its efficacy and safety profile. Their research findings shed light on the notable impact of Imatinib on modulating inflammatory mark- ers, such as IL-6, within the patient population afflicted with COVID-19 (Emadi et al. 2020). The impact of cur- cumin-piperine supplementation on inflammatory mark- ers, oxidative stress, clinical parameters, and mortality rates among critically ill patients in the ICU afflicted with COVID-19 was rigorously investigated by Askari et al. in a study conducted in Iran. Their comprehensive findings illuminated the favorable effects of curcumin-piperine supplementation, manifesting in the normalization of key clinical indicators including fever, blood oxygen satura- tion, and respiratory rate. Additionally, their research
indicated that the utilization of curcumin-piperine sup- plementation exerted significant influence on important clinical outcomes, encompassing the duration of ICU stay, mortality rates, as well as the levels of prominent biomarkers such as CRP, ESR, alanine aminotransferase (ALT), aspartate aminotransferase (AST), LDH, and cre- atinine (Askari et al. 2021). These findings contribute to the body of scientific knowledge about potential thera- peutic interventions targeting inflammation and clinical outcomes in COVID-19 patients, aligning with the focus of the present study.
In a separate investigation conducted by Beigmoham- madi et al. in Iran, the impact of vitamins A, B, C, D, and E on patient improvement and mortality rates within the context of COVID-19 was systematically evaluated. Their study illuminated the beneficial effects associated with vitamin consumption, particularly the modulation of key serum biomarkers including WBC count, CRP, ESR rate, IL-6, interferon-gamma (IFN-γ), and TNF-α (Beigmohammadi et al. 2020). These findings contribute valuable insights into the potential therapeutic role of supplementation in mitigating inflammatory responses and improving clinical outcomes among COVID-19 patients, aligning with the overarching objective of our investigation.
The findings of the present study have elucidated a notable disparity in mortality rates, whereby 31% of patients within the control group and 8.5% within the intervention group succumbed to the disease. This obser- vation underscores the substantial impact of a 14-day regimen of NBS natural supplements in dramatically diminishing mortality rates among individuals afflicted with COVID-19. The mortality rate in the control group was substantially higher than in the intervention group, emphasizing the potential life-saving effects of NBS supplementation. While this study was not designed to explore the exact mechanisms underlying the reduction in mortality, it is plausible that the modulation of inflam- mation and immune response by NBS played a role in improving patient outcomes. One possible mechanism of NBS’s action is its rich content of vitamins, minerals, and essential fatty acids. These nutrients are known to play roles in immune function and inflammation modulation. The diverse array of bioactive molecules in NBS may act synergistically to enhance the body’s defense mechanisms against the virus. Further research is needed to elucidate the specific mechanisms by which NBS exerts its effects.
The striking congruence observed in the outcomes of the present clinical trial and the antecedent investigations conducted by Jalilian et al. (Azizi Jalilian et al. 2022) and Mosadegh (Mosadegh et al. 2022a, b) attests to the robust and consistent efficacy of NBS superfood as an adjunc- tive therapeutic agent amidst the backdrop of distinct
SARS-CoV-2 variants. It is of paramount significance to underscore the temporal context in which these studies transpired. While Jalilian’s inquiry centered on the alpha variant and Mosadegh’s exploration delved into the delta variant, the current trial unfolded amidst the tumultuous surge of the Omicron variant. The unwaveringly affirma- tive findings across this spectrum of viral strains lead us to a pivotal revelation: NBS’s mechanisms of action tran- scend the taxonomic boundaries of microorganisms, even in the face of the relentless mutational dynamics of emerging variants. This inherent adaptability delineates NBS as a unique and conceivably indispensable asset in the battle against infectious diseases, effectively counter- ing the vexing challenges posed by the dynamic evolution of surface antigens in novel variants and the emergence of resistance phenomena. A foundational principle mer- iting recognition in the context of emerging viral variants resides in the potential attenuation of vaccine efficacy, frequently ascribed to the emergence of novel surface antigens and the subsequent evolution of antibody-resist- ant strains. These concerns have engendered a global reconsideration of therapeutic strategies, underscoring the urgency of innovative interventions. The extraor- dinary uniformity in the outcomes of the investigations by Jalilian, Mosadegh, and our present study under- scores NBS as a beacon of optimism amidst these com- plex circumstances. It defies the conventional paradigms of pathogen-specific therapeutic modalities, presenting itself as a versatile and adaptable solution to the cease- lessly shifting landscape of viral variants. This profound attribute of NBS warrants further comprehensive exami- nation and inquiry, as it may harbor the key to ameliorat- ing the devastating consequences of emerging infectious diseases, fortifying our resilience in the face of the capri- cious forces of microbial evolution. As we confront the perpetual arms race between human scientific ingenuity and the adaptive abilities of viral entities, NBS emerges as a steadfast ally, offering a glimpse into the trajectory of pandemic preparedness and therapeutic innovation. The main limitation of the present study was budget limita- tion. The present study was a pilot study with a budget limitation. Therefore, we could not evaluate more bio- markers such as the levels of interleukins among included patients.
In conclusion, the present study has elucidated the
capacity of NBS superfood supplement to effectively mit- igate inflammatory markers in critically ill ICU patients afflicted by ARDS caused by the Omicron variant of COVID-19. These results underscore the potential util- ity of NBS superfood as an adjunct to standard antiviral treatments for inflammation reduction and the attenua- tion of mortality rates in COVID-19 patients.
Acknowledgements
We would like to express our heartfelt gratitude to Ali Teimoori, Peyman Eini, Fatemeh Torkamanasadi, and Somayeh Shokri for their invaluable contribu- tions to this article. Their dedication, expertise, and collaborative spirit have significantly enriched the research and content of this work. We are deeply appreciative of their commitment and support throughout the research pro- cess, which has played a pivotal role in bringing this article to fruition.
Author contributions
- M., A. K. and Y. E.: conceptualization; data curation; formal analysis; and writ- ing—original draft. Y. E., M. N., S. H. H., F. A. J.: conceptualization; methodology; project administration; and writing—original draft. M. M., E. A., R. A., G. K.: data curation; formal analysis; writing—original draft; and writing—review & edit- ing. F. A. J., N. A., S. M.: language editing.
Funding
This research did not receive any specific grant from funding agencies in public, commercial, or nonprofit organizations.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The study protocols adhered to the principles outlined in the Helsinki Declara- tion, with all procedures receiving official validation from the Ethics Commit- tee of Hamadan University of Medical Sciences (Protocol Number: IR.UMSHA. REC.1401.1043; Approval Date: 2023-03-04). Additionally, the study’s protocol has been registered in the Iranian Registry of Clinical Trials (IRCT), accessible
at www.irct.ir (IRCT Registration Number: IRCT20230116057135N2; Registra- tion Date: 2023-04-05). The aims of the study were explained to patients. A questionnaire was prepared for each of the patients and a written informed consent was acquired from all patients.
Consent for publication
Not applicable.
Competing interests
All of the authors declare that there are no commercial, personal, political, or any other potential conflicting interests related to the submitted manuscript.
Received: 6 January 2024 Accepted: 12 March 2024
References
Ahmed AU (2011) An overview of inflammation: mechanism and conse- quences. Front Biol 6(4):274–281
Askari G, Alikiaii B, Soleimani D, Sahebkar A, Mirjalili M, Feizi A, Iraj B, Bagherniya M (2021) Effect of curcumin-pipeine supplementation on clinical status, mortality rate, oxidative stress, and inflammatory markers in critically ill ICU patients with COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials 22(1):1–3
Azimi T, Hamidi-Farahani R, Asgari A, Rajabi J, Ahmadi M, Darvishi M, Aminian- far M, Naghoosi H, Soleiman-Meigooni S (2021) Molecular detection and clinical characteristics of bacterial and viral main etiological agents caus- ing respiratory tract infections in Tehran, Iran. Gene Rep 24:101267
Azizi Jalilian F, Keshavarz G, Khazaei S, Nezamdoost M, Hashemi SH, Mamani M, Ansari N, Amini R, Khalkhali A, Keshavarz A (2022) The effects of nutrition bio-shield superfood powder on immune system function: a clinical trial study among patients with COVID-19. Front Immunol 13:919402
Bayat A, Khalkhali A, Mahjoub AR (2014) Nutrition bio-shield superfood: healthy and live herbal supplement for immune system enhancement. Int J Nutr Food Eng 15(1):6–9
Beigmohammadi MT, Bitarafan S, Hoseindokht A, Abdollahi A, Amoozadeh L,
- Mahmoodi Ali Abadi and M. Foroumandi, (2020) Impact of vitamins A,
B, C, D, and E supplementation on improvement and mortality rate in ICU patients with coronavirus-19: a structured summary of a study protocol for a randomized controlled trial. Trials 21(1):614
Beigmohammadi MT, Bitarafan S, Hoseindokht A, Abdollahi A, Amoozadeh L, Soltani D (2021) The effect of supplementation with vitamins A, B, C, D, and E on disease severity and inflammatory responses in patients with COVID-19: a randomized clinical trial. Trials 22(1):1–9
Emadi A, Chua JV, Talwani R, Bentzen SM, Baddley J (2020) Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials 21(1):1–5
Fan Y, Li X, Zhang L, Wan S, Zhang L, Zhou F (2022) SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal Transduct Target Ther 7(1):141
Gadotti AC, de Castro Deus M, Telles JP, Wind R, Goes M, Ossoski RGC, de Padua AM, de Noronha L, Moreno-Amaral A, Baena CP (2020) IFN-γ is an independent risk factor associated with mortality in patients with moder- ate and severe COVID-19 infection. Virus Res 289:198171
Gönen MS, Alaylıoğlu M, Durcan E, Özdemir Y, Şahin S, Konukoğlu D, Nohut OK, Ürkmez S, Küçükece B, Balkan İİ (2021) Rapid and effective vitamin D supplementation may present better clinical outcomes in COVID-19 (SARS-CoV-2) patients by altering serum INOS1, IL1B, IFNg, cathelicidin- LL37, and ICAM1. Nutrients 13(11):4047
Jafarzadeh A, Jafarzadeh S, Nozari P, Mokhtari P, Nemati M (2021) Lymphopenia an important immunological abnormality in patients with COVID-19: possible mechanisms. Scand J Immunol 93(2):e12967
Kandeel M, Mohamed ME, Abd El-Lateef HM, Venugopala KN, El-Beltagi HS (2022) Omicron variant genome evolution and phylogenetics. J Med Virol 94(4):1627–1632
Kuwahara H, Horie T, Ishikawa S, Tsuda C, Kawakami S, Noda Y, Kaneko T, Tahara S, Tachibana T, Okabe M (2010) Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy. Free Radi- cal Biol Med 48(9):1252–1262
Le TT, Andreadakis Z, Kumar A, Román RG, Tollefsen S, Saville M, Mayhew S (2020) The COVID-19 vaccine development landscape. Nat Rev Drug Discov 19(5):305–306
Legacy M, Seely D, Conte E, Psihogios A, Ramsay T, Fergusson DA, Kanji S, Simmons J-G, Wilson K (2022) Dietary supplements to reduce symptom severity and duration in people with SARS-CoV-2: study protocol for a randomised, double-blind, placebo controlled clinical trial. BMJ Open 12(3):e057024
Leulseged TW, Hassen IS, Ayele BT, Tsegay YG, Abebe DS, Edo MG, Maru EH, Zewde WC, Naylor LK, Semane DF (2021) Laboratory biomarkers of COVID-19 disease severity and outcome: findings from a developing country. PLoS ONE 16(3):e0246087
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet 395(10229):1033–1034
Meo S, Meo A, Al-Jassir F, Klonoff D (2021) Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci 25(24):8012–8018
Mortaz E, Tabarsi P, Jamaati H, Dalil Roofchayee N, Dezfuli NK, Hashemian SM, Moniri A, Marjani M, Malekmohammad M, Mansouri D (2021) Increased serum levels of soluble TNF-α receptor is associated with ICU mortality in COVID-19 patients. Front Immunol 12:592727
Mosadegh M, Khalkhali A, Erfani Y, Nezamdoost M (2022a) The effect of Nutrition Bio-shield superfood (NBS) on disease severity and laboratory biomarkers in patients with COVID-19: a randomized clinical trial. Microb Pathog 172:105792
Mosadegh M, Khalkhali A, Sadeghi Y, Erfani Y (2022b) The anti-inflammatory effects of the nutrition bio-shield (NBS) supplement intake on adjuvant- induced rheumatoid arthritis in rat. Turk J Immunol 10(2):95–101
Oristrell J, Oliva JC, Casado E, Subirana I, Domínguez D, Toloba A, Balado A, Grau M (2022) Vitamin D supplementation and COVID-19 risk: a population-based, cohort study. J Endocrinol Invest 45:167–179
Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Plan- chais C, Porrot F, Robillard N, Puech J (2021) Reduced sensitivity of SARS- CoV-2 variant Delta to antibody neutralization. Nature 596(7871):276–280
Ruan Q, Yang K, Wang W, Jiang L, Song J (2020) Clinical predictors of mortal- ity due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 46(5):846–848
تعداد بازدید : 298




نظرات دیگران؛